Guillain barre syndrome yellow fever vaccine information
Home » » Guillain barre syndrome yellow fever vaccine informationYour Guillain barre syndrome yellow fever vaccine images are available. Guillain barre syndrome yellow fever vaccine are a topic that is being searched for and liked by netizens today. You can Find and Download the Guillain barre syndrome yellow fever vaccine files here. Download all royalty-free photos and vectors.
If you’re looking for guillain barre syndrome yellow fever vaccine pictures information linked to the guillain barre syndrome yellow fever vaccine interest, you have visit the ideal blog. Our site frequently provides you with suggestions for viewing the highest quality video and picture content, please kindly hunt and find more enlightening video articles and images that fit your interests.
Guillain Barre Syndrome Yellow Fever Vaccine. Viscerotropic complications following the vaccine are termed yellow fever vaccine-associated viscerotropic disease YEL-AVD. VAERS is a passive reporting system. Yellow fever vaccine is highly effective approaching 100. Severe adverse reaction to yellow fever YF vaccine includes the yellow fever vaccine-associated neurotropic disease.
 Scielo Brazil Brazilian Recommendations On The Safety And Effectiveness Of The Yellow Fever Vaccination In Patients With Chronic Immune Mediated Inflammatory Diseases Brazilian Recommendations On The Safety And Effectiveness Of The From scielo.br
Scielo Brazil Brazilian Recommendations On The Safety And Effectiveness Of The Yellow Fever Vaccination In Patients With Chronic Immune Mediated Inflammatory Diseases Brazilian Recommendations On The Safety And Effectiveness Of The From scielo.br
In 2004 the Brighton Collaboration BC was commissioned as a vaccine safety research network to develop standardized case definitions for AEFI 8. Viscerotropic complications following the vaccine are termed yellow fever vaccine-associated viscerotropic disease YEL-AVD. Safety of meningococcal B vaccines. The WHO lists the following symptoms for the Guillain-Barré syndrome. This terminology includes postvaccinal encephalitis acute disseminated encephalomyelitis and Guillain-Barré syndrome. Guillain-Barré syndrome and vaccination.
Vaccines and Guillain-Barré Syndrome The notion that Guillain-Barre syndrome GBS could be a consequence of vaccination was born of the swine influenza vaccine program administered in the United States in 1976.
The 17D vaccine which is based on a live attenuated viral strain is the only commercially available yellow fever vaccine. Mucocutaneous Lymph Node Syndrome etiology Neisseria meningitidis Serogroup B immunology Rotavirus Vaccines adverse effects Vaccines adverse effects. Guillain-Barré syndrome and vaccination. Modus operandi of the committee and additional information. Yellow fever YF can be prevented by an attenuated vaccine YEL. Thus influenza vaccines prevent GBS by protecting against natural influenza infection.
 Source: jns-journal.com
Source: jns-journal.com
Guillain-Barré syndrome GBS is a rare disorder where the bodys immune system damages nerve cells causing muscle weakness and sometimes paralysis. Another rare reaction to yellow fever vaccine is yellow fever vaccine-associated neurologic disease. This includes several conditions such as meningoencephalitis inflammation of the brain and its membranes Guillain-Barré syndrome acute disseminated encephalomyelitis inflammation of the brain and spinal cord and bulbar palsy paralysis of the motor units of the cranial nerves. The first symptoms include weakness or tingling sensation which usually start in the legs and can spread to the arms and face. As GuillainBarre Syndrome GBS or acute disseminated encephalomyelitis ADEM.
 Source: mdpi.com
Source: mdpi.com
While its cause is not fully understood the syndrome often follows infection with a virus or bacteria. Vaccines and Guillain-Barré Syndrome The notion that Guillain-Barre syndrome GBS could be a consequence of vaccination was born of the swine influenza vaccine program administered in the United States in 1976. Guillain-Barré syndrome GBS is a rare disorder where the bodys immune system damages nerve cells causing muscle weakness and sometimes paralysis. Influenza vaccines reduce the risk of influenza infection which causes Guillain-Barré Syndrome GBS. Viscerotropic complications following the vaccine are termed yellow fever vaccine-associated viscerotropic disease YEL-AVD.
 Source: thelancet.com
Source: thelancet.com
As GuillainBarre Syndrome GBS or acute disseminated encephalomyelitis ADEM. The first symptoms include weakness or tingling sensation which usually start in the legs and can spread to the arms and face. Guillain-Barré syndrome acute disseminated encephalomyelitis bulbar palsy and Bell palsy. This terminology includes postvaccinal encephalitis acute disseminated encephalomyelitis and Guillain-Barré syndrome. Other travel related vaccines may be given.
 Source: nature.com
Source: nature.com
Yellow fever YF can be prevented by an attenuated vaccine YEL. On Monday the Food and Drug Administration announced the new warning flagging reports of Guillain-Barre syndrome an immune system disorder that. The clinical manifestations included in YEL-AND are meningoencephalitis neurotropic disease Guillain-Barré syndrome GBS and acute disseminated encephalomyelitis ADEM 7. While its cause is not fully understood the syndrome often follows infection with a virus or bacteria. It is given as a single subcutaneous or intramuscular injection.
 Source: researchgate.net
Source: researchgate.net
At the time the estimated risk of GBS following receipt of the swine flu vaccine was estimated to be about 1 per 100000 recipients. At the time the estimated risk of GBS following receipt of the swine flu vaccine was estimated to be about 1 per 100000 recipients. In 2004 the Brighton Collaboration BC was commissioned as a vaccine safety research network to develop standardized case definitions for AEFI 8. Modus operandi of the committee and additional information. Summary of all reported YEL AVD cases post Stamaril vaccination.
 Source: sussextravelclinic.com
Source: sussextravelclinic.com
Viscerotropic complications following the vaccine are termed yellow fever vaccine-associated viscerotropic disease YEL-AVD. Each year in the United States an estimated 3000 to 6000 people develop GBS. The 17D vaccine which is based on a live attenuated viral strain is the only commercially available yellow fever vaccine. There is no medicine to treat or cure yellow fever. Nervous system reactions such as inflammation of the brain encephalitis andor spinal cord covering meningitis or Guillain-Barré Syndrome GBS among others.
 Source: mdpi.com
Source: mdpi.com
Guillain-Barré syndrome GBS is a rare disorder where the bodys immune system damages nerve cells causing muscle weakness and sometimes paralysis. Another rare reaction to yellow fever vaccine is yellow fever vaccine-associated neurologic disease. As yellow fever vaccine or separated by a 4 week interval. Other travel related vaccines may be given. In the past it occurred mainly in infants as encephalitis10 leading to a.
 Source: nps.org.au
Source: nps.org.au
Yellow fever vaccine is highly effective approaching 100. In 2004 the Brighton Collaboration BC was commissioned as a vaccine safety research network to develop standardized case definitions for AEFI 8. We reviewed neurologic adverse events AE following YEL that were reported to the national Vaccine Adverse Events Reporting System VAERS. Other travel related vaccines may be given. Yellow fever vaccine is highly effective approaching 100.
 Source: thelancet.com
Source: thelancet.com
Rotavirus vaccines and Kawasaki disease. Safety of immunization in immunocompromised individuals. This includes several conditions such as meningoencephalitis inflammation of the brain and its membranes Guillain-Barré syndrome acute disseminated encephalomyelitis inflammation of the brain and spinal cord and bulbar palsy paralysis of the motor units of the cranial nerves. In very rare cases yellow fever vaccination can cause encephalitis and is possibly associated with Guillain-Barre Syndrome and acute disseminated encephalomyelitis ADEM. As yellow fever vaccine or separated by a 4 week interval.
 Source: scielo.br
Source: scielo.br
At the time the estimated risk of GBS following receipt of the swine flu vaccine was estimated to be about 1 per 100000 recipients. On Monday the Food and Drug Administration announced the new warning flagging reports of Guillain-Barre syndrome an immune system disorder that. Other travel related vaccines may be given. Safety of meningococcal B vaccines. Yellow fever vaccine is highly effective approaching 100.
 Source: ijidonline.com
Source: ijidonline.com
Guillain-Barre Syndrome etiology. Safety of live 14-14-2 Japanese encephalitis vaccine. Yellow Fever Vaccine Safety Data in the General Population 12 Appendix 3. VAERS is a passive reporting system. The first symptoms include weakness or tingling sensation which usually start in the legs and can spread to the arms and face.
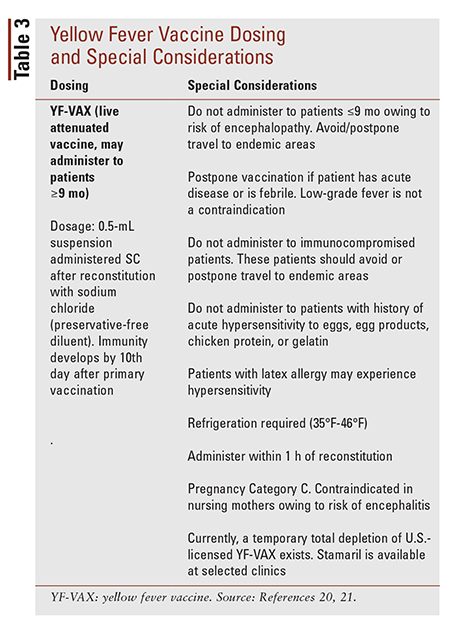 Source: uspharmacist.com
Source: uspharmacist.com
Nervous system reactions such as inflammation of the brain encephalitis andor spinal cord covering meningitis or Guillain-Barré Syndrome GBS among others. As GuillainBarre Syndrome GBS or acute disseminated encephalomyelitis ADEM. VAERS is a passive reporting system. At the time the estimated risk of GBS following receipt of the swine flu vaccine was estimated to be about 1 per 100000 recipients. Modus operandi of the committee and additional information.
Source:
Yellow Fever Vaccine Safety Data in Contraindicated Groups 17 Appendix 4. The clinical manifestations included in YEL-AND are meningoencephalitis neurotropic disease Guillain-Barré syndrome GBS and acute disseminated encephalomyelitis ADEM 7. Yellow fever vaccine is highly effective approaching 100. Guillain-Barré syndrome GBS is a rare disorder where the bodys immune system damages nerve cells causing muscle weakness and sometimes paralysis. Modus operandi of the committee and additional information.
Source:
While its cause is not fully understood the syndrome often follows infection with a virus or bacteria. For some people these symptoms can lead to paralysis of the legs arms or muscles in the face. Influenza vaccines reduce the risk of influenza infection which causes Guillain-Barré Syndrome GBS. The first symptoms include weakness or tingling sensation which usually start in the legs and can spread to the arms and face. Yellow fever vaccine can prevent yellow fever.
 Source: healthsmartvaccines.com
Source: healthsmartvaccines.com
The first symptoms include weakness or tingling sensation which usually start in the legs and can spread to the arms and face. Thus influenza vaccines prevent GBS by protecting against natural influenza infection. Other travel related vaccines may be given. As GuillainBarre Syndrome GBS or acute disseminated encephalomyelitis ADEM. Safety of yellow fever vaccines.
Source:
Another rare reaction to yellow fever vaccine is yellow fever vaccine-associated neurologic disease. In 2004 the Brighton Collaboration BC was commissioned as a vaccine safety research network to develop standardized case definitions for AEFI 8. Yellow fever is a serious disease caused by the yellow fever virus. Safety of immunization in immunocompromised individuals. The clinical manifestations included in YEL-AND are meningoencephalitis neurotropic disease Guillain-Barré syndrome GBS and acute disseminated encephalomyelitis ADEM 7.
Source:
Guillain-Barré syndrome and vaccination. This includes several conditions such as meningoencephalitis inflammation of the brain and its membranes Guillain-Barré syndrome acute disseminated encephalomyelitis inflammation of the brain and spinal cord and bulbar palsy paralysis of the motor units of the cranial nerves. We reviewed neurologic adverse events AE following YEL that were reported to the national Vaccine Adverse Events Reporting System VAERS. This terminology includes postvaccinal encephalitis acute disseminated encephalomyelitis and Guillain-Barré syndrome. However influenza vaccines can very rarely cause Guillain-Barré Syndrome GBS within 6 weeks of vaccination in adults at an estimated rate of 1-3 cases per.
 Source: researchgate.net
Source: researchgate.net
Summary of all reported YEL AVD cases post Stamaril vaccination. Safety of yellow fever vaccines. The first symptoms include weakness or tingling sensation which usually start in the legs and can spread to the arms and face. Yellow Fever Vaccine Safety Data in Contraindicated Groups 17 Appendix 4. However influenza vaccines can very rarely cause Guillain-Barré Syndrome GBS within 6 weeks of vaccination in adults at an estimated rate of 1-3 cases per.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title guillain barre syndrome yellow fever vaccine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
Category
Related By Category
- Child tax credit payments bill information
- Child tax credit july 2021 portal information
- Finance of america stock information
- Team usa in olympics information
- Mel gibson christ movie information
- Child tax credit portal down information
- Stephen a smith i dont care gif information
- Usa basketball in olympics information
- Social security yuba city information
- Stephen a smith megan olivi information